Exploring the electronic structure of aqueous solutions with soft x-ray spectroscopy
Water and aqueous solutions are probably the most essential substances in our lives. Consequently, understanding their properties and functions on a molecular level are a central research topic that touches upon technology, physics, chemistry, and biology. Also, it turns out to be highly challenging!
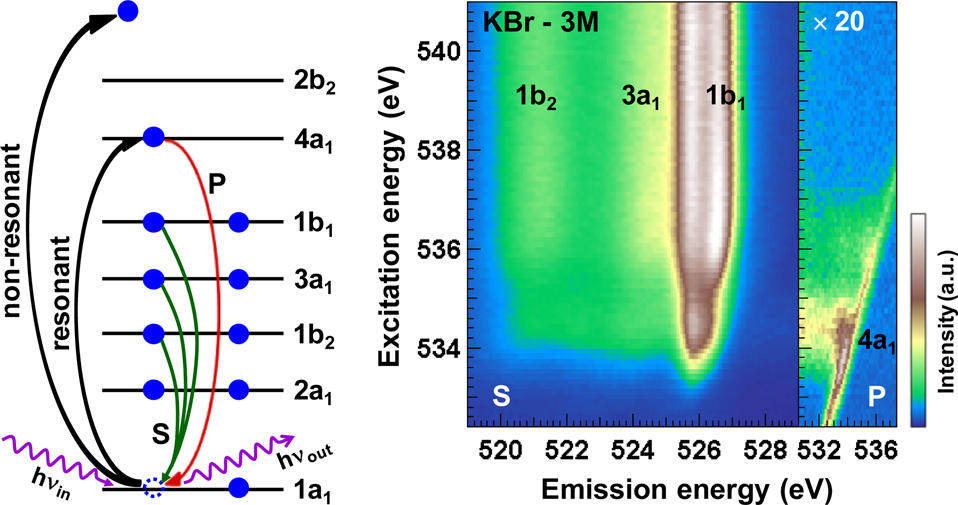
Over the last decade, researchers at the Institute for Photon Science and Synchrotron Radiation (IPS) at the Karlsruhe Institute of Technology (KIT), in close collaboration with the University of Heidelberg, the University of Würzburg, and the University of Nevada, Las Vegas (UNLV), have pioneered the use of soft x-ray spectroscopy (see Fig. 1, left) to study such liquid systems. The electronic structure of the liquids and solutions and, in particular, the interaction between the different constituents is in the focus of these studies. In three recent publications [1–3], the team was able to study such interactions in aqueous ammonia and a variety of salt solutions. These studies open up the possibility of probing hydrogen bonds in aqueous solutions by combining x-ray emission spectroscopy (XES) and resonant inelastic soft x-ray scattering (RIXS).
The experiments are very challenging, since the measurement techniques require a high-flux soft x-ray beamline and an experimental station that typically features an ultra-high vacuum (UHV) environment. To nevertheless be able to study liquids and systems at ambient pressures, the team uses the dedicated Solid and Liquid Spectroscopic Analysis (SALSA) endstation [4], which is run by UNLV and the IPS in the framework of a collaboration agreement. SALSA is operated at beamline 8.0.1 of the Advanced Light Source (ALS), and the KIT activities have recently been highlighted in a feature article at the ALS (https://als.lbl.gov/exploring-structure-aqueous-solutions-salsa/). This work also forms the basis for developing in situ and operando soft x-ray spectroscopy techniques to be used at the X-SPEC beamline of the IPS, currently in commissioning at the KIT.
A particular strength of the SALSA setup is its high-transmission soft x-ray spectrometer [5], which allows combining soft x-ray emission and absorption spectroscopy by collecting a dense grid of emission spectra within a standard x-ray absorption scan. This results in a two-dimensional intensity map (RIXS map) that can be used to extract both (resonant) emission and absorption spectra.
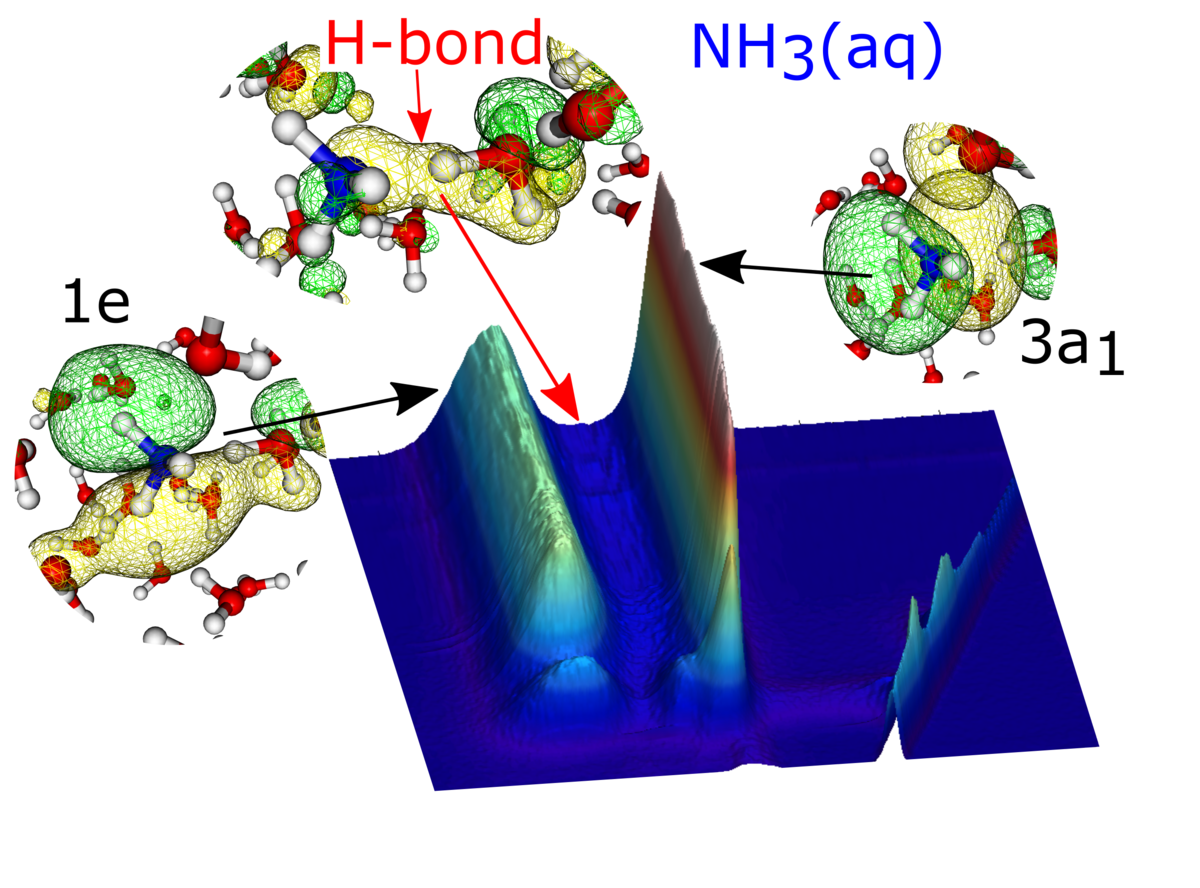
In the ammonia study [2], the researchers compared the gas phase system (i.e., without hydrogen bonding) to ammonia in aqueous solution (i.e., strongly hydrogen-bonded). It was possible to identify a clear signature of the covalent part of the hydrogen bonds as “new” intensity in the RIXS map of the liquid (Fig. 2), shedding light on the molecular interactions in the aqueous environment.
The experiments on salts [1,3] represent an important step towards understanding the local interactions in the so-called “Hofmeister series”. This series describes ion-specific effects on the solubility of proteins in aqueous solutions, which play a central role in biology. A specific order of the ions according to their ability to increase or decrease the protein solubility can be found, but the detailed explanation is still a mystery. The recent studies are able to identify ion-specific impacts on the interaction between ions and water and thus build the foundation for studying systems of increasing complexity, i.e., solutions of molecules of increasing size and co-solutions of salts with amino acids, peptides, and, ultimately, proteins.
[1] Y. L. Jeyachandran, F. Meyer, S. Nagarajan, A. Benkert, M. Bär, M. Blum, W. Yang, F. Reinert, C. Heske, L. Weinhardt, and M. Zharnikov, J. Phys. Chem. Lett. 5, 4143 (2014).
[2] L. Weinhardt, E. Ertan, M. Iannuzzi, M. Weigand, O. Fuchs, M. Bär, M. Blum, J. D. Denlinger, W. Yang, E. Umbach, M. Odelius, and C. Heske, Phys. Chem. Chem. Phys. 17, 27145 (2015).
[3] Y. L. Jeyachandran, F. Meyer, A. Benkert, M. Bär, M. Blum, W. Yang, F. Reinert, C. Heske, L. Weinhardt, and M. Zharnikov, J. Phys. Chem. B 120, 7687 (2016).
[4] M. Blum, L. Weinhardt, O. Fuchs, M. Bär, Y. Zhang, M. Weigand, S. Krause, S. Pookpanratana, T. Hofmann, W. Yang, J. D. Denlinger, E. Umbach, and C. Heske, Rev. Sci. Instrum. 80, 123102 (2009).
[5] O. Fuchs, L. Weinhardt, M. Blum, M. Weigand, E. Umbach, M. Bär, C. Heske, J. Denlinger, Y. D. Chuang, W. McKinney, Z. Hussain, E. Gullikson, M. Jones, P. Batson, B. Nelles, and R. Follath, Rev. Sci. Instrum. 80, 63103 (2009).